生体システム論研究室MEグループでは末梢血管剛性インデックスによる新しい交感神経活動評価法の研究に取り組んでいますが,Frontiers in Physiologyに新たな論文が掲載されました.この論文は,MEグループの修了生である穴井 怜志君の修士研究を許 自強先生が発展させたものです.おめでとうございます!
引き続き,末梢血管剛性インデックスを用いて人間の自律神経系を非侵襲的に評価する研究を進めていきたいと思っています.よろしくお願いします.
Noninvasive Characterization of Peripheral Sympathetic Activation across Sensory Stimuli Using a Peripheral Arterial Stiffness Index
Ziqiang Xu, Reiji Anai, Harutoyo Hirano, Zu Soh and Toshio Tsuji
Frontiers in Physiology (Computational Physiology and Medicine), 14:1294239, DOI: 10.3389/fphys.2023.1294239, PUBLISHED 08 January 2024. (SCI, IF=4.0)
URL: https://www.frontiersin.org/articles/10.3389/fphys.2023.1294239/full
PDF: https://www.readcube.com/articles/10.3389/fphys.2023.1294239
<論文内容>
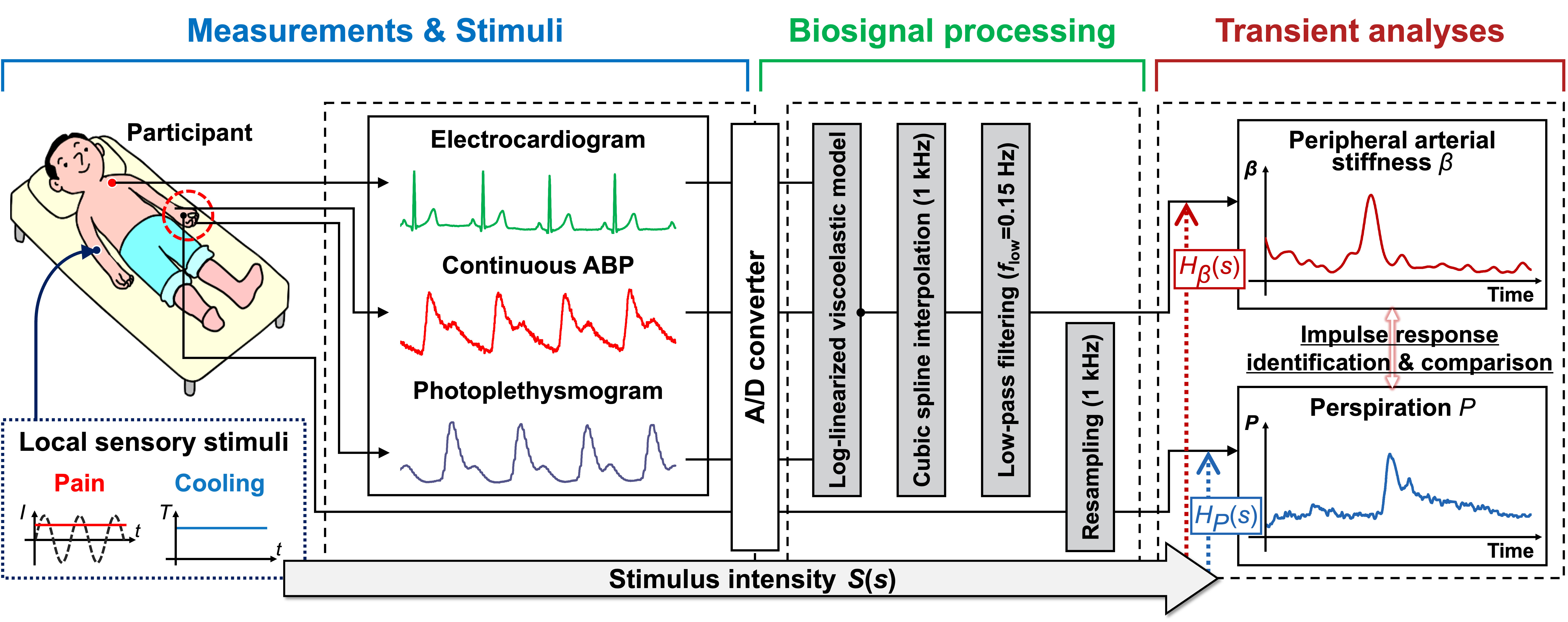
The peripheral arterial stiffness index has been proposed and validated as a noninvasive measure quantifying stimulus intensity based on amplitude changes induced by sympathetic innervation of vascular tone. However, its temporal response characteristics remain unclear, thus hindering continuous and accurate monitoring of the dynamic process of sympathetic activation. This paper presents a study aimed at modeling the transient response of the index across sensory stimuli to characterize the corresponding peripheral sympathetic activation.
The index was measured using a continuous arterial pressure monitor and a pulse oximeter during experiments with local pain and local cooling stimuli designed to elicit different patterns of sympathetic activation. The corresponding response of the index was modeled to clarify its transient response characteristics across stimuli.
The constructed transfer function accurately depicted the transient response of the index to local pain and local cooling stimuli (Fit percentage: 78.4% ± 11.00% and 79.92% ± 8.79%). Differences in dead time (1.17 ± 0.67 and 0.99 ± 0.56 s, p = 0.082), peak time (2.89 ± 0.81 and 2.64 ± 0.68 s, p = 0.006), and rise time (1.81 ± 0.50 and 1.65 ± 0.48 s, p = 0.020) revealed different response patterns of the index across stimuli. The index also accurately characterized similar vasomotor velocities at different normalized peak amplitudes (0.19 ± 0.16 and 0.16 ± 0.19 a.u., p = 0.007).
Our findings flesh out the characterization of peripheral arterial stiffness index responses to different sensory stimuli and demonstrate its validity in characterizing peripheral sympathetic activation. This study valorizes a noninvasive method to characterize peripheral sympathetic activation, with the potential to use this index to continuously and accurately track sympathetic activators.
末梢血管剛性インデックスは交感神経支配によって誘発される振幅変化に基づいて刺激強度を定量化可能な非侵襲的尺度として提案され,検証されている.しかし,その時間的反応特性は不明であるため,交感神経活性化の動的過程の連続的で正確なモニタリングの妨げとなっている.本論文では,感覚刺激に対する指標の過渡応答をモデル化し,対応する末梢交感神経の活性化を評価することを目的とした.
交感神経活性化の異なるパターンを誘発するようにデザインされた局所疼痛刺激と局所冷却刺激による実験中に,連続血圧モニタとパルスオキシメータを用いて末梢血管剛性インデックスを測定した.刺激に対する応答特性を明らかにするために,末梢血管剛性インデックスの反応を伝達関数でモデル化した.
同定された伝達関数は,局所疼痛刺激および局所冷却刺激に対する指標の過渡応答を正確に描写した(適合率:78.4%±11.00%および79.92%±8.79%).むだ時間(1.17±0.67秒と0.99±0.56秒、p = 0.082),ピーク時間(2.89±0.81秒と2.64±0.68秒,p = 0.006),立ち上がり時間(1.81±0.50秒と1.65±0.48秒,p = 0.020)の違いから,刺激によって末梢血管剛性インデックスの反応パターンが異なることが明らかになった.この指標はまた,異なる正規化ピーク振幅(0.19±0.16および0.16±0.19 a.u.,p = 0.007)において,同様の血管運動速度を正確に特徴付けた.
我々の所見は,異なる感覚刺激に対する末梢血管剛性反応の特徴をより具体化し,末梢交感神経活性化の特徴付けにおける有効性を実証した.この研究は末梢の交感神経活性化を特徴づける非侵襲的な方法を評価するものであり,この指標を用いて交感神経活性化因子を継続的かつ正確に追跡できる可能性がある.
──これまでの末梢血管剛性インデックスに関する主要な研究成果─────
Prediction of blood pressure changes during surgical incision using the minimum evoked current of vascular stiffness value under sevoflurane anesthesia
Daiki Shorin, Satoshi Kamiya, Ryuji Nakamura, Ayaka Ishibashi, Noboru Saeki, Toshio Tsuji, and Yasuo M. Tsutsumi
Scientific Reports, volume 13, Article number: 20486, doi:10.1038/s41598-023-46942-y, Published online: 22 November 2023. (SCI, IF=4.6)
Beat-to-beat Estimation of Peripheral Arterial Stiffness from Local PWV for Quantitative Evaluation of Sympathetic Nervous System Activity
Ziqiang Xu, Toshiki Sakagawa, Akira Furui, Shumma Jomyo, Masanori Morita, Masamichi Ando, and Toshio Tsuji
IEEE Transactions on Biomedical Engineering, Volume: 69, Issue: 9, pp. 2806-2816, Digital Object Identifier: 10.1109/TBME.2022.3154398 , September 2022 (SCI, IF=4.756)
Prediction of blood pressure change during surgical incision under opioid analgesia using sympathetic response evoking threshold
Satoshi Kamiya, Ryuji Nakamura, Noboru Saeki, Takashi Kondo, Hirotsugu Miyoshi, Soushi Narasaki, Atsushi Morio, Masashi Kawamoto, Harutoyo Hirano, Toshio Tsuji, and Yasuo M Tsutsumi
Scientific Reports, volume 11, Article number: 9558, doi.org/10.1038/s41598-021-87636-7, Published online: 5 May 2021. (SCI, IF=3.998)
Cardiorespiratory Synchronisation and Systolic Blood Pressure Correlation of Peripheral Arterial Stiffness During Endoscopic Thoracic Sympathectomy
Toshifumi Muneyasu, Harutoyo Hirano, Akira Furui, Zu Soh, Ryuji Nakamura, Noboru Saeki, Yoshiyuki Okada, Masashi Kawamoto, Masao Yoshizumi, Atsuo Yoshino, Takafumi Sasaoka, Shigeto Yamawaki, and Toshio Tsuji
Scientific Reports, volume 11, Article number: 5966, doi.org/10.1038/s41598-021-85299-y, Published online: 16 March 2021. (SCI, IF=3.998)
Peripheral Arterial Stiffness During Electrocutaneous Stimulation is Positively Correlated with Pain-related Brain Activity and Subjective Pain Intensity: An fMRI Study
Toshio Tsuji, Fumiya Arikuni, Takafumi Sasaoka, Shin Suyama, Takashi Akiyoshi, Zu Soh, Harutoyo Hirano, Ryuji Nakamura, Noboru Saeki, Masashi Kawamoto, Masao Yoshizumi, Atsuo Yoshino, and Shigeto Yamawaki
Scientific Reports, volume 11, Article number: 4425, doi.org/10.1038/s41598-021-83833-6, Published online: 24 February 2021. (SCI, IF=3.998)
Estimation of Arterial Viscosity Based on an Oscillometric Method and Its Application in Evaluating the Vascular Endothelial Function
Hiroshi Tanaka, Akihisa Mito, Harutoyo Hirano, Zu Soh, Ryuji Nakamura, Noboru Saeki, Masashi Kawamoto, Yukihito Higashi, Masao Yoshizumi, and Toshio Tsuji
Scientific Reports, volume 9, Article number: 2609, doi:10.1038/s41598-019-38776-4, Published online: 22 February 2019. (SCI, IF=4.259)
A new arterial mechanical property indicator reflecting differences in invasive stimulus intensity induced by alteration of remifentanil concentration during laryngoscopy
Kensuke Yanabe, Ryuji Nakamura, Noboro Saeki, Elbegzaya Sukhdorj, Abdugheni Kutluk, Hiroki Hirano, Harutoyo Hirano, Masao Yoshizumi, Toshio Tsuji, and Masashi Kawamoto
Minerva Anestesiologica, Volume: 84, Issue: 3, pp. 311-318, DOI: 10.23736/S0375-9393.17.11796-7, Published: MAR, 2018. (SCI, IF=2.623)
Quantitative Evaluation of Pain during Electrocutaneous Stimulation using a Log-Linearized Peripheral Arterial Viscoelastic Model
Hiroki Matsubara, Hiroki Hirano, Harutoyo Hirano, Zu Soh, Ryuji Nakamura, Noboru Saeki, Masashi Kawamoto, Masao Yoshizumi, Atsuo Yoshino, Takafumi Sasaoka, Shigeto Yamawaki, and Toshio Tsuji
Scientific Reports, volume 8, Article number: 3091, doi:10.1038/s41598-018-21223-11, Published online: 15 February 2018 (SCI, IF=4.259).
Alteration of Arterial Mechanical Impedance Greater than that of Photoplethysmogram and Laser Doppler Flowmetry during Endoscopic Thoracic Sympathectomy
Elbegzaya Sukhdorj, Ryuji Nakamura, Noboro Saeki, Kensuke Yanabe, Abdugheni Kutluk, Hiroki Hirano, Harutoyo Hirano, Toshio Tsuji, and Masashi Kawamoto
Journal of Medical and Biological Engineering, Volume 37, Issue 6, pp. 820-825, DOI: 10.1007/s40846-017-0246-0, DEC 2017 (SCI, IF=1.018)
───────────────────────────────────